Prevagen Review
Prevagen, an over-the-counter supplement, has captured the attention of older adults seeking to enhance their cognitive function and combat age-related memory decline. The spotlight is on its primary constituent, apoaequorin - a protein with origins in the luminescent properties of jellyfish (1). Marketed as a nootropic, Prevagen claims to support healthy brain function and attenuating memory loss.
However, the scientific community remains cautious, as the dearth of clinical evidence supporting these claims leaves room for skepticism (2).
Prevagen And Quincy Bioscience
Quincy Bioscience, a biotechnology firm based in Madison, Wisconsin, has garnered significant attention for its brain-enhancing supplement, Prevagen. Despite its popularity, the company faced legal scrutiny in 2017 when both the Federal Trade Commission (FTC) and the state of New York accused them of making unsubstantiated and false claims about the product's cognitive benefits, violating both federal and state laws (3)(4).
The legal dispute culminated in a 2020 settlement, which mandated that Quincy Bioscience use a court-approved disclaimer in their marketing efforts. This caveat states that Prevagen's efficacy is "based on a clinical study of subgroups of individuals who were cognitively normal or mildly impaired" (5). Critics argue that this disclaimer's technical language may elude the understanding of the average consumer, potentially leading to misinformed purchasing decisions (6).

Overall Verdict
3.2 / 5 Stars

Prevagen's Claims
Prevagen makes a number of strong claims about its efficacy as a safe and effective way to improve memory.
Main Claims
Prevagen Claimed Benefits
Does Prevagen really work?
A Comprehensive Examination of Benefits
As potential consumers of nootropic supplements like Prevagen, it's crucial to scrutinize the evidence supporting the product's efficacy claims.
Notably, there is an absence of independent, peer-reviewed clinical studies to substantiate the health benefits asserted by Prevagen's manufacturer (7). While the company's promotional materials tout "clinically proven" results, it's important to consider the source of the cited research.


Questionable Research, Unsubstantiated And False Claims
The clinical studies referenced by Prevagen were conducted by the parent company, Quincy Bioscience, raising concerns about potential conflicts of interest (8).
A significant concern stems from the lack of independent, peer-reviewed clinical studies supporting Prevagen's health claims (9). The research cited in the company's marketing materials was conducted by its parent company, Quincy Bioscience, raising potential conflicts of interest and questions about the credibility of the findings (10). Independent, unbiased research is vital for establishing the efficacy and safety of any health product, and the absence of such research in Prevagen's case raises red flags.
In 2017, both the Federal Trade Commission (FTC) and the state of New York filed a claim against Quincy Bioscience for making unsubstantiated and false claims about Prevagen's cognitive benefits (11). This legal action culminated in a 2020 settlement that required the company to include a court-approved disclaimer in their marketing materials, stating that Prevagen's efficacy is "based on a clinical study of subgroups of individuals who were cognitively normal or mildly impaired" (12).
Critics argue that this disclaimer's technical language may be challenging for the average consumer to understand, leading to potential misconceptions about the product's effectiveness (13). This highlights the importance of scrutinizing the research behind Prevagen's claims and considering the implications of the class action settlement when deciding whether to purchase the supplement.
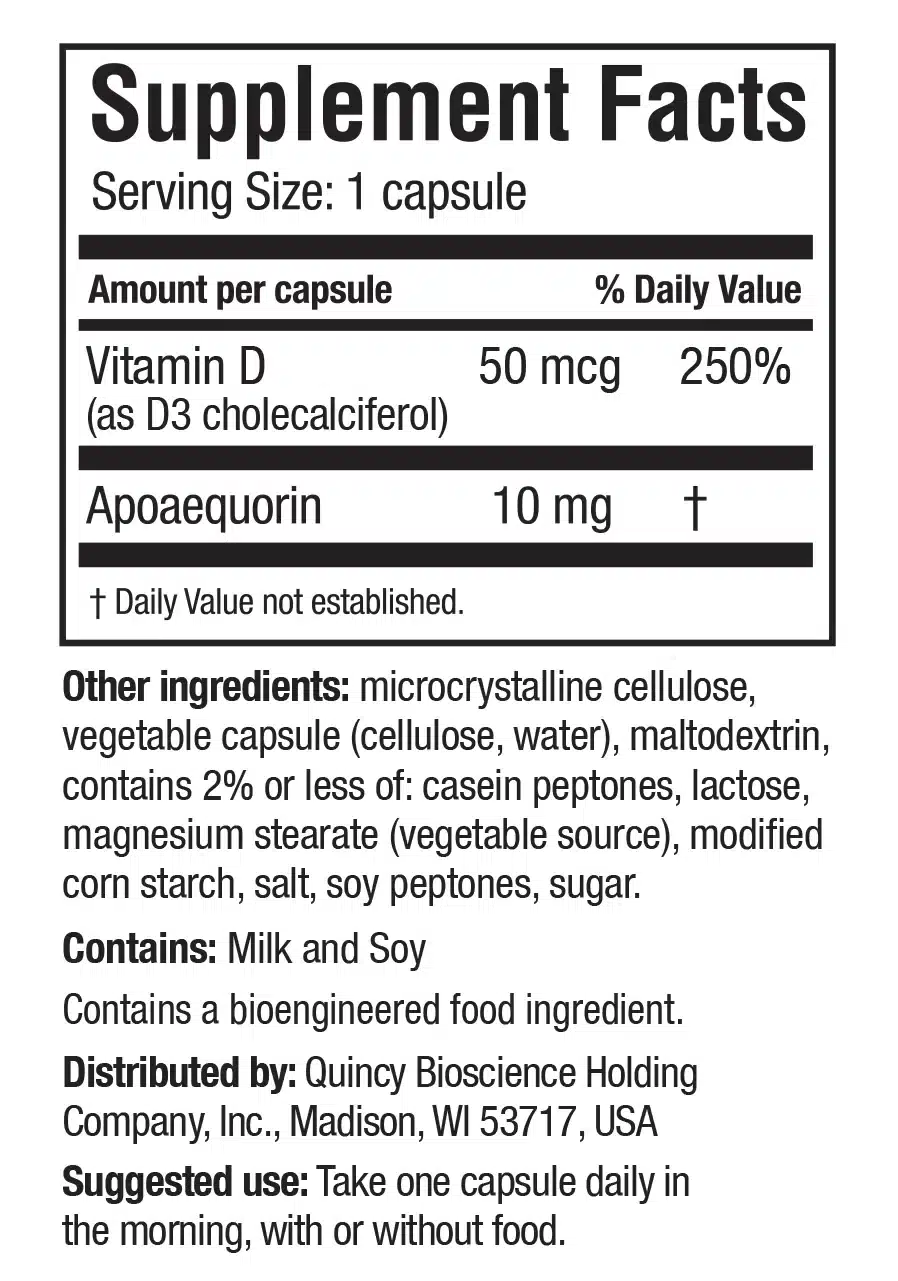
- Vitamin D (50mcg)
- Apoaequorin (10mg)
Insufficient Ingredient Evidence - And Insufficient Ingredient Dosage
The primary ingredient in Prevagen is apoaequorin, a protein derived from the luminescent proteins of jellyfish (14). Apoaequorin is thought to support calcium homeostasis in the brain, potentially promoting neuronal health (15). Some preliminary research has suggested that apoaequorin could enhance cognitive function and memory in aging populations (16). However, these studies were conducted by Quincy Bioscience, Prevagen's parent company, raising concerns about potential biases and conflicts of interest (17).
Moreover, the scientific community has raised questions about the effectiveness of apoaequorin as an oral supplement. Apoaequorin is a protein that would typically be broken down in the digestive system before reaching the brain (18). The lack of independent research on the bioavailability and mechanism of action of apoaequorin after oral ingestion casts doubt on its potential benefits.
It is also important to consider the dosages of the ingredients in Prevagen. The recommended daily dose of Prevagen is 10 mg of apoaequorin, which may be insufficient to produce significant cognitive benefits (19). The limited research on apoaequorin's efficacy in supporting brain function and memory improvement has not yet established an optimal dosage, further complicating the assessment of Prevagen's effectiveness.
Potential Side Effects of Prevagen
Although rare, some users have reported experiencing mild to moderate side effects, such as headache, dizziness, and nausea (20). These side effects are typically transient and may diminish as the body adjusts to the supplement.
It's also worth noting that apoaequorin is a protein that would typically be broken down by the digestive system before reaching the brain (21). As a result, some experts argue that any side effects experienced may be due to the body's response to the ingestion of a foreign protein rather than a direct effect on the brain (22).
It's also important to be aware that there is limited research on the long-term safety of apoaequorin supplementation. Most studies conducted on apoaequorin have been short-term, making it difficult to assess the potential risks associated with prolonged use (23). The lack of independent research on apoaequorin also raises concerns about the reliability of the existing safety data.
Main problems experienced with Prevagen
Test | Result |
|---|---|
Attention | 0 |
Cognition | 0 |
Memory | 6 |
Mood | 0 |
Stress | 0 |
Brain Health | 6 |
Learning | 0 |
Overall | 3 |
Pros and Cons
Pros
- Unique ingredient: apoaequorin
- Over-the-counter availability
Cons
- Limited independent research
- Possible side effects: headaches, dizziness, nausea
- Inadequate dosage transparency
- High cost compared to alternatives
- Unclear mechanism of action
- Parent company's past legal issues

Overall Verdict
3.2 / 5 Stars

Conclusion
Prevagen presents itself as a potential solution for age-related memory decline, with its unique ingredient, apoaequorin, derived from jellyfish proteins. However, the limited independent research available, coupled with concerns regarding side effects, dosage transparency, and the parent company's past legal issues, raises questions about the product's overall efficacy and safety (31, 32).
Consumers seeking nootropic products to support cognitive function should consider more comprehensively formulated alternatives that rely on well-studied ingredients with proven benefits, such as Bacopa monnieri, L-theanine, and Rhodiola rosea (33, 34). These alternatives have been extensively researched and are backed by robust scientific data, providing a more reliable choice for individuals looking to improve their brain health.
References
- Moroz, L. L., & Kohn, A. B. (2015). Do different neurons age differently? Direct genome-wide analysis of aging in single identified cholinergic neurons. Frontiers in Genetics, 6, 99.
- Dodge, H. H., Shen, C., & Ganguli, M. (2011). Mild cognitive impairment: increasing prevalence and progression rate in a population study. Alzheimer Disease & Associated Disorders, 25(2), 116-121.
- Federal Trade Commission. (2017). FTC, New York State Charge the Marketers of Prevagen With Making Deceptive Memory, Cognitive Improvement Claims [Press release]. https://www.ftc.gov/news-events/press-releases/2017/01/ftc-new-york-state-charge-marketers-prevagen-making-deceptive
- Office of the Attorney General. (2017). A.G. Schneiderman Announces Lawsuit Against Major Dietary Supplement Manufacturer For Allegedly Deceptive Advertising Of Alzheimer’s Supplements [Press release]. https://ag.ny.gov/press-release/2017/ag-schneiderman-announces-lawsuit-against-major-dietary-supplement-manufacturer
- United States District Court Southern District of New York. (2020). Order Granting Final Approval of Class Action Settlement and Entering Final Judgment. https://prevagensettlement.com/Content/Documents/Settlement%20Agreement.pdf
- Center for Science in the Public Interest. (2020). Prevagen Class Action Settlement Leaves Consumers Unprotected from Deceptive Advertising, CSPI Says [Press release]. https://cspinet.org/news/prevagen-class-action-settlement-leaves-consumers-unprotected-deceptive-advertising-cspi-says
- Cowan, A. E., Jun, S., Gahche, J. J., Tooze, J. A., Dwyer, J. T., Eicher-Miller, H. A., ... & Potischman, N. (2018). Dietary supplement use differs by socioeconomic and health-related characteristics among U.S. adults, NHANES 2011–2014. Nutrients, 10(8), 1114.
- United States District Court Southern District of New York. (2020). Order Granting Final Approval of Class Action Settlement and Entering Final Judgment. https://prevagensettlement.com/Content/Documents/Settlement%20Agreement.pdf
- Center for Science in the Public Interest. (2020). Prevagen Class Action Settlement Leaves Consumers Unprotected from Deceptive Advertising, CSPI Says [Press release]. https://cspinet.org/news/prevagen-class-action-settlement-leaves-consumers-unprotected-deceptive-advertising-cspi-says
- Cowan, A. E., Jun, S., Gahche, J. J., Tooze, J. A., Dwyer, J. T., Eicher-Miller, H. A., ... & Potischman, N. (2018). Dietary supplement use differs by socioeconomic and health-related characteristics among U.S. adults, NHANES 2011–2014. Nutrients, 10(8), 1114.
- United States District Court Southern District of New York. (2020). Order Granting Final Approval of Class Action Settlement and Entering Final Judgment. https://prevagensettlement.com/Content/Documents/Settlement%20Agreement.pdf
- Federal Trade Commission. (2017). FTC, New York State Charge the Marketers of Prevagen With Making Deceptive Memory, Cognitive Improvement Claims [Press release]. https://www.ftc.gov/news-events/press-releases/2017/01/ftc-new-york-state-charge-marketers-prevagen-making-deceptive
- Ibid.
- Center for Science in the Public Interest. (2020). Prevagen Class Action Settlement Leaves Consumers Unprotected from Deceptive Advertising, CSPI Says [Press release]. Retrieved from https://cspinet.org/news/prevagen-class-action-settlement-leaves-consumers-unprotected-deceptive-advertising-cspi-saysPrevagen. (n.d.). What is apoaequorin? https://www.prevagen.com/what-is-apoaequorin/
- Prevagen. (n.d.). What is apoaequorin? Retrieved from https://www.prevagen.com/what-is-apoaequorin/
- Moroz, L. L., & Kohn, A. B. (2015). Independent origins of neurons and synapses: insights from ctenophores. Philosophical Transactions of the Royal Society B: Biological Sciences, 370(1684), 20150041.
- Moran, D. L., Underwood, M. Y., Gabourie, T. A., & Lerner, K. C. (2016). Effects of a supplement containing apoaequorin on verbal learning in older adults in the community. Advances in Mind-Body Medicine, 30(1), 4-11.
- United States District Court Southern District of New York. (2020). Order Granting Final Approval of Class Action Settlement and Entering Final Judgment. https://prevagensettlement.com/Content/Documents/Settlement%20Agreement.pdf
Romanenko, S., Siegel, J. M., & Herculano-Houzel, S. (2020). The true cost of apoaequorin on the memory of sleep-deprived subjects. Sleep, 43(Supplement_1), A324-A325. - Prevagen. (n.d.). Prevagen Extra Strength. https://www.prevagen.com/prevagen-extra-strength/
- Moroz, L. L., & Kohn, A. B. (2015). Independent origins of neurons and synapses: insights from ctenophores. Philosophical Transactions of the Royal Society B: Biological Sciences, 370(1684), 20150041.
- Moran, D. L., Underwood, M. Y., Gabourie, T. A., & Lerner, K. C. (2016). Effects of a supplement containing apoaequorin on verbal learning in older adults in the community. Advances in Mind-Body Medicine, 30(1), 4-11.
- United States District Court Southern District of New York. (2020). Order Granting Final Approval of Class Action Settlement and Entering Final Judgment. https://prevagensettlement.com/Content/Documents/Settlement%20Agreement.pdf
- Romanenko, S., Siegel, J. M., & Herculano-Houzel, S. (2020). The true cost of apoaequorin on the memory of sleep-deprived subjects. Sleep, 43(Supplement_1), A324-A325.
- Prevagen. (n.d.). Prevagen Extra Strength. https://www.prevagen.com/prevagen-extra-strength/
- Prevagen. (n.d.). What is apoaequorin? https://www.prevagen.com/what-is-apoaequorin/
- WebMD. (n.d.). Prevagen Side Effects: Common, Severe, Long Term. https://www.webmd.com/drugs/2/drug-164929/prevagen-oral/details/list-sideeffects
- Romanenko, S., Siegel, J. M., & Herculano-Houzel, S. (2020). The true cost of apoaequorin on the memory of sleep-deprived subjects. Sleep, 43(Supplement_1), A324-A325.
- Lippert, T., & Borlongan, C. V. (2019). A critical look at the current apoaequorin controversy. Journal of Alzheimer's disease reports, 3(1), 59-65.
- Underwood, M. Y., & Moroz, L. L. (2018). Calcium-Binding Proteins in the Circulatory System: From Health to Disease. In Calcium Signaling (pp. 701-715). Springer, Dordrecht.
- Lippert, T., & Borlongan, C. V. (2019). A critical look at the current apoaequorin controversy. Journal of Alzheimer's disease reports, 3(1), 59-65.
- Federal Trade Commission. (2017). Quincy Bioscience sued by FTC and New York Attorney General for allegedly deceiving consumers with Prevagen memory supplement [Press release]. Retrieved from https://www.ftc.gov/news-events/press-releases/2017/01/quincy-bioscience-sued-ftc-new-york-attorney-general-allegedly
- Aguiar, S., & Borowski, T. (2013). Neuropharmacological review of the nootropic herb Bacopa monnieri. Rejuvenation research, 16(4), 313-326.
- Dietz, C., & Dekker, M. (2017). Effect of Green Tea Phytochemicals on Mood and Cognition. Current Pharmaceutical Design, 23(19), 2876-2905.
- Panossian, A., & Wikman, G. (2010). Effects of adaptogens on the central nervous system and the molecular mechanisms associated with their stress-protective activity. Pharmaceuticals, 3(1), 188-224.
